

Insulin Pen InspectionReliable Detection of Assembly Errors, Quality Assurance
Automation Improves Efficiency and Patient Safety
Insulin Pen Inspection
The incidence of diabetes worldwide is increasing all the time. Insulin pens allow patients to manage their therapy themselves. During the manufacturing process, full inspection of pen assembly is essential to ensure a high degree of patient safety.
With VITRONIC, you check that each assembly step has been completed correctly. This is done directly after each step, so that you can detect any therapy-relevant defects that will no longer be visible in the final assembled product.
Identify All Defects
When inspecting insulin pens, it is important to detect assembly errors before final assembly and exclude defective component groups from manufacturing as early as possible. Due to the fast cycle rates, automated visual inspection (AVI) is the only option for a 100% inspection.
VITRONIC solutions are also designed so that, even with multi-line assembly, full inspection is possible while still minimizing the hardware costs of camera stations. You also benefit from the modular approach to covering the scope of inspection. The inspection can be performed after each assembly step in the process or following the assembly of a component group.
- 100% inspectioneven at max. cycle time
Insulin Pen Inspection—Areas
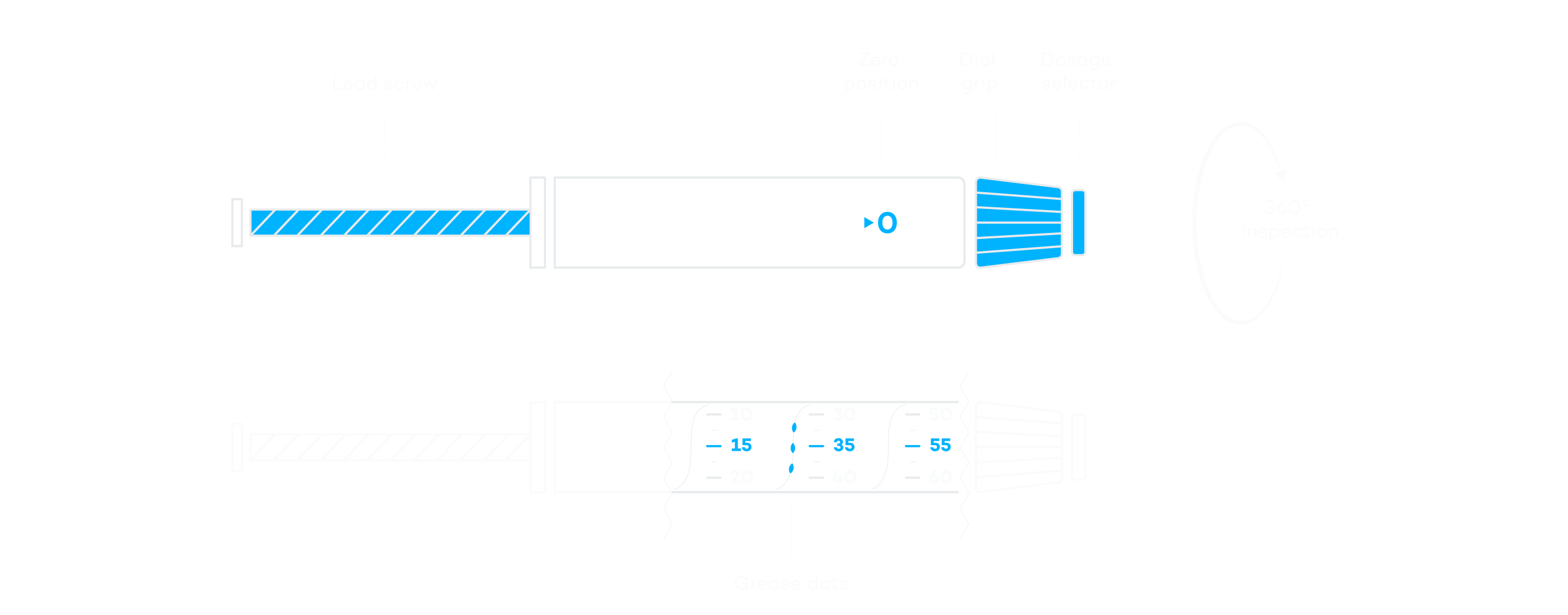

Reduce the Risk with Automated 100% Inspection
As a manufacturer of insulin pens, patient safety is your top priority. Which is why you need to guarantee the functionality and operating safety of each pen. The products must be flawless and ready to use. A comprehensive inspection ensures that no deviations occur in the assembly of the pen or the geometry of the components.
In other words, 100% defect detection optimizes product safety, thereby contributing to maximum patient safety.
Qualification Package – Play It Safe
You can integrate the VINSPEC HEALTHCARE AVI system for insulin pens seamlessly into your assembly line. The solution uses perfectly coordinated hardware and software, plus an adapted sensor design. The intelligent algorithms in VITRONIC software are specifically adjusted to meet the requirements of each inspection task thanks to easy-to-use parameters. This optimizes inspection performance.





