Menü

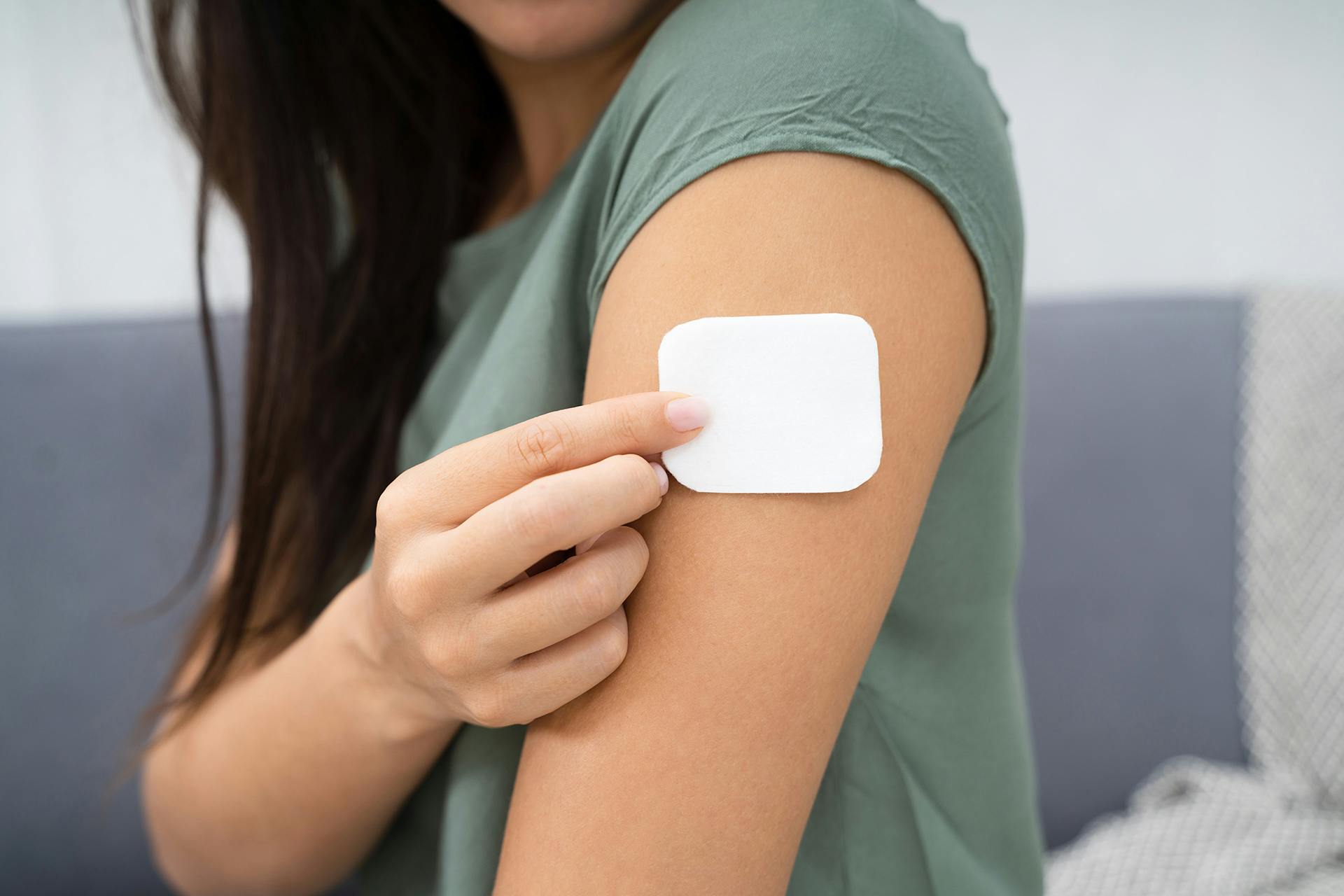
Transdermal Patch Inspection Convenient Therapy That's Safe Too!
Inspect Transdermal Patches Safely and Efficiently
Transdermal Patch Inspection
For Taking Away and Passing On
Brochure
VINSPEC HEALTHCARE—Inline Pharmaceutical/Medical Device Inspection
Brochure
VINSPEC HEALTHCARE—for Transdermal Therapeutic Systems