Menü

Blow-Fill-Seal Inspection Automated Inspection Reduces Costs
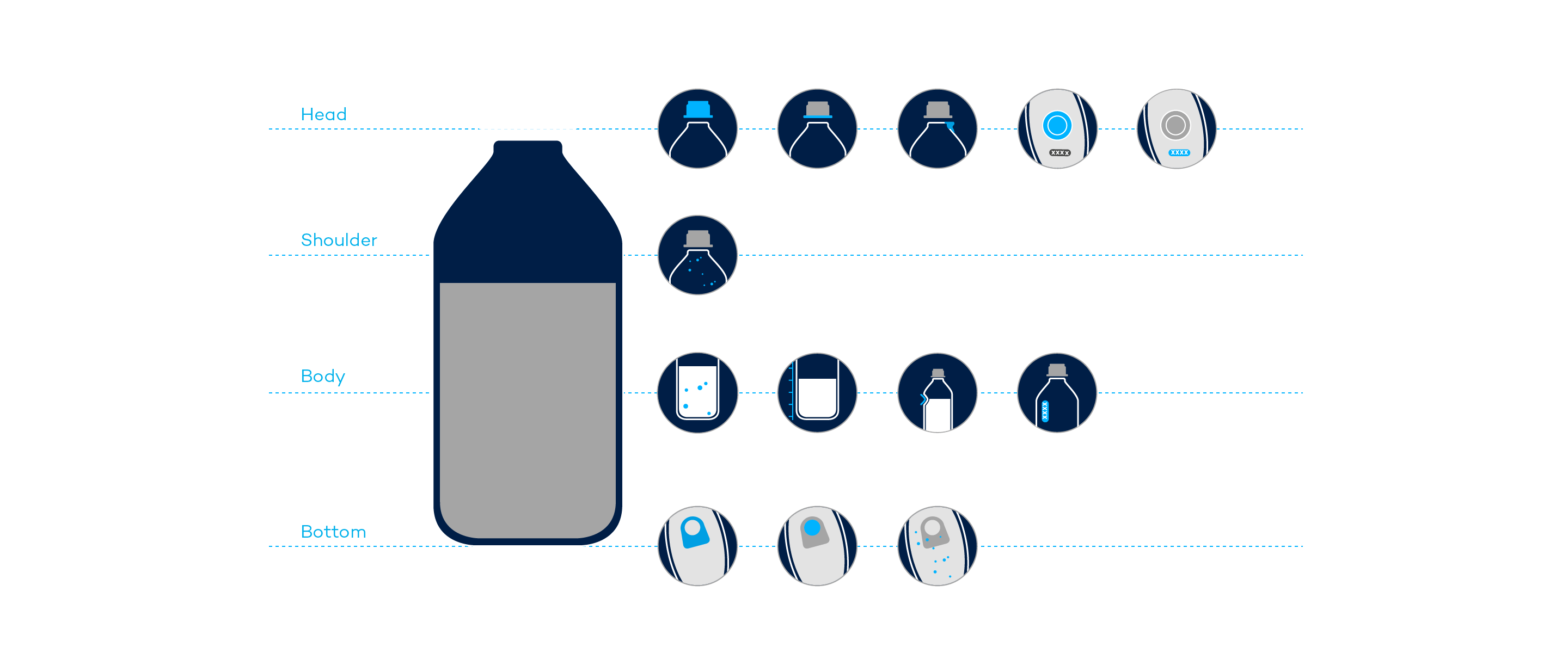
Visual Quality Inspection for BFS Containers
Blow-Fill-Seal Inspection
For Taking Away and Passing On
Brochure
VINSPEC HEALTHCARE for Blow-Fill-Seal-Containers
Brochure
VINSPEC HEALTHCARE - Inline Pharmaceutical/Medical Device Inspection